Necrotizing Enterocolitis (NEC)
Necrotizing Enterocolitis (NEC) is the most frequent and life-threatening disorder that affects the intestine of premature infants. More than 5% of premature infants with very-low-birth-weight (<1,500g) develop NEC with an average mortality rate of 25%. The survivors are often faced with long-term neurological complications. Biomarkers to identify infants at risk to develop NEC are not available. Treatment options are very limited, and surgical removal of the affected intestinal regions is often unavoidable.
Clinical observations led to the hypothesis that HMO reduce NEC risk
Epidemiological data shows that human milk-fed infants are at a much lower risk to develop NEC than formula-fed infants. This obeservation led us to hypothesize that HMO that are highly abundant in human milk but not in infant formula contribute to the lower NEC risk in human milk-fed infants.
Preclinical studies identified a specific HMO structure-benefit relationship
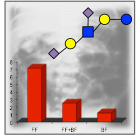
We tested this hypothesis by isolating HMO from human milk and assessing their in vivo efficacy in an established preclinical rat model of the disease. Once we confirmed that HMO improved survival and reduced NEC pathology, we used a multi-dimensional chromatography approach to identify the most effective HMO in the preclinical model and collaborated with the UCSD Glycotechnology Core to elucidate its structure (Jantscher-Krenn et al. 2012).
Clinical cohort studies confirmed preclinical results
Results from preclinical studies are often controversial because animal models rarely fully recapitulate human development, anatomy and physiology. To overcome this challenge, we designed a multicenter cohort study with sites across North America that recruited mothers and their preterm infants and collected several thousand milk samples. We then used our new rapid, high-throughput technology to analyze HMO composition and identified associations between concentrations of individual HMO and the occurrence of NEC. We found that infants that develop NEC received less of a specific HMO with the milk than infants that did not develop NEC. The specific HMO was the same as the one we had identified earlier to be protective against NEC in the preclinical model. Preclinical data and clinical cohort data matched!
Combined results inform clinical intervention studies
Matching results from preclinical studies (Jantscher-Krenn et al. 2012) and now two independent cohort studies (van Niekerk et al. 2014, Autran et al. submitted) help inform the design of a clinical intervention study to prove that the specific HMO indeed reduces NEC incidence.
Synthesis approaches and medicinal chemistry enable clinical intervention studies
New approaches to HMO synthesis and medicinal chemistry (Hu et al. 2014) are going to make HMO and even more potent HMO derivatives available to enable clinical intervention studies.
Elucidating the mechanism
Using the preclinical model, we continue to elucidate the underlying mechanism of how the specific HMO reduces NEC pathology. Understanding the mechanism is going to provide additional information about NEC etiology and pathogenesis and help develop new treatment options and feeding recommendations.
Transferring the same approach to other clinical contexts
A similar approach of combining preclinical studies and human cohort studies to identify HMO structure-function relationships and inform human intervention studies can be applied to a variety of clinical contexts related to maternal and infant health.
The project is supported in part by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), Abbott Nutrition and Friesland Campina Domo.